Atoms are the basic units of matter and the defining structure of elements. The term "atom" comes from the Greek word for indivisible, because it was once thought that atoms were the smallest things in the universe and could not be divided. We now know that atoms are made up of three particles: protons, neutrons and electrons — which are composed of even smaller particles, such the quarks.
Atoms were created after the Big Bang 13.7 billion years ago. As the hot, dense new universe cooled, conditions became suitable for quarks and electrons to form. Quarks came together to form protons and neutrons, and these particles combined into nuclei. This all took place within the first few minutes of the universe's existence, according to CERNAnd the things that you can’t see too – such as air, cells of your body are also made up of atoms.
Now, I hope you guys understand that what an atom can be basically. Next, let’s understand the structure of an atom.
An atom is a naturally charged particle, which means there two types of charges in it, negative and positive charges with equal quantity.
Subatomic Particles in an Atom
There are three subatomic particles in an atom electron, proton, neutron. But only two particles have an electric charge. The proton has a positive electric charge and the electron have a negative electric charge. And there is no charge in neutron means it is chargeless
Because protons are not fundamental particles, they possess a measurable size; the root means square charge radius of a proton is about 0.84–0.87 FM (or 0.84×10−15 to 0.87×10−15 m).
The size of a neutron is about the size of the proton. But The electron is 20000 times lighter than the proton and neutron.
As we all know the unlike charges attract each other. Because the protons and neutrons have unlike charges so they also attract each other. But neutron has no charge so no one will attract it
The electrons orbit its nucleus with almost with the speed of light, like in the solar system the planets orbit the sun but with a different speed
The Nucleus of an Atom
A cluster of protons and neutrons makes up the nucleus of an atom. The most of weight of an atom is concentrated on the nucleus, but it takes up a very small amount volume of the atom.
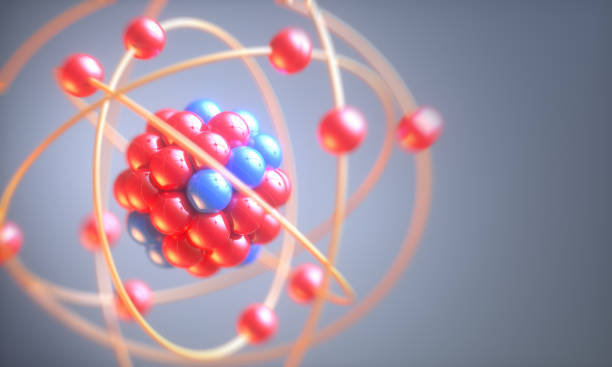
In an atom, The 99.99 percentage of space is empty. For example, consider an atom with the size of a football in a football stadium, Then the center of the atom would be the size of a pea in the center of the stadium.
Usually, in an atom the number of protons and electrons will be equal, That’s what makes an atom naturally charged. Some big atoms have more than 100 of both.
The electrons are arranged in layers according to their energy level around the nucleus known as electron shells. Like satellites revolve around the earth. Bigger atoms have more layers than smaller ones.
These revolving electron helps to make a bond with another atom and to make a molecule.
The arrangement of eight electrons in the outermost shells of atoms is called octet electron configuration. To become more stable the atoms leave or take electrons for their outermost shells.
Making Molecules
Atoms can make bonds/molecules. A molecule can be made up of the same atoms or they can be different from each other. Gases such as hydrogen have simple molecules made of just two atoms, while plastic can be made from endlessly repeating molecules made up of thousands of atoms joined in very long lines
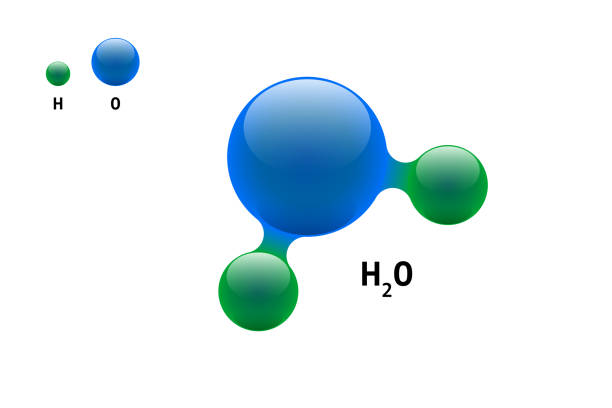
e.g. The molecule of water (H2O) is made from two atoms of hydrogen and one atom of oxygen.
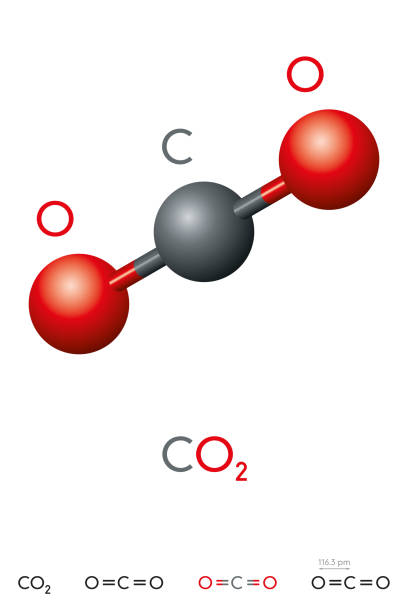
The molecule of carbon dioxide (CO2) is made up of two oxygen atoms and one atom of carbon.
Atomic Bonds
Atoms bind together to make molecules with the help of their electrons. They take, give or share their electrons. There are three main types of atomic bonds.
Ionic Bond
In this bond, one atom gives or takes electrons to another atom to become more stable. After giving the electron the first atom becomes positive and the atom which takes the electron became negative And because of these, unlike charges they attract each other and develop a bond between them.

e.g. consider there is an atom with 1 electron in its outermost shell, and another atom with 7 electrons in its outermost shell then what will the first atom do, it will just give one electron to another atom that has 7 electrons in its outermost shell. And then both atoms will get 8 electrons in their outermost shell.
Covalent Bond
In this bond, two atoms share their electrons.
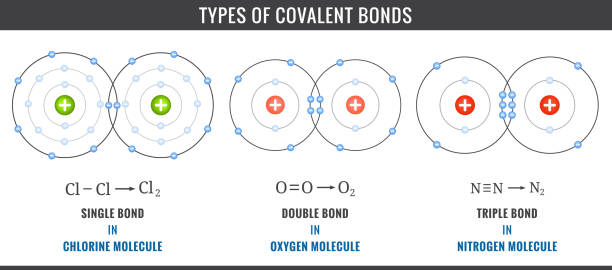
e.g. consider there are two atoms with 7 electrons in the outermost shell. They will share their one electron as shown in the above picture. And then both atoms will get 8 electrons in their outermost shell
Metallic Bond

In this bond, a large number of atoms share their electron as shown in the above picture, and make a large cloud of bonds.
Something Amazing
Hey, but a question arises here that the nucleus of an atom is made up of proton and neutron but what are they can be made of?
Let me tell you that the protons and neutrons are made up of smaller particles called quarks.
There can be 3 or more quarks in each neutron and proton. But what about the quarks. What are they made up of?

Many scientists believe that everything in-universe is made from the energy, vibration of matter, or by very small vibrational particles called string. Our scientists also think that the string ar founded in the quarks. known as string theory.
But so far no one knows that what are the strings made up of.


Post a Comment