Welcome to your latest scientific rendezvous, thoughtfully curated by the team at freeastroscience.com. We're here to unravel the mystery of a unique protein structure, known as the "rippled beta sheet." This structure, first predicted in 1953, has remained largely theoretical until now. Join us as we delve into the recent findings about this unusual protein configuration and its promising potential for future developments.
Unlocking the Enigma: The Rippled Beta Sheet Protein Structure
In a groundbreaking revelation, scientists have successfully created and detailed the elusive "rippled beta sheet" protein structure in a lab setting, employing sophisticated X-ray crystallography. This landmark accomplishment, published in the esteemed Chemical Science journal in July, is poised to catalyze the strategic design of distinctive materials based on this unique architectural blueprint.
The New Frontier in Structural Protein Designs
Jevgenij Raskatov, an acclaimed associate professor of chemistry and biochemistry at UC Santa Cruz, and the paper's corresponding author, lauds this discovery as a stepping stone towards the structure-oriented design of unparalleled molecular constructs. This paves the way for potential advancements in both material development and biomedical applications, broadening the horizon of protein engineering.
An Array of Shapes and Sizes: The World of Proteins
Proteins, the building blocks of life, display an impressive array of shapes and sizes, each tailored to serve distinct structural and functional roles within living cells. Amongst these, certain structural patterns such as the alpha helix are ubiquitous in numerous protein structures.
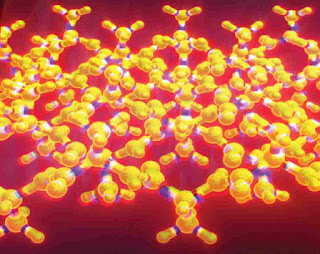
The "rippled sheet" is a nuanced variation of the pleated beta sheet, a renowned structural pattern found in countless proteins. This intriguing concept of the rippled beta sheet was first proposed by Linus Pauling and Robert Corey back in 1953, just two years after they introduced the pleated beta sheet. Despite its initial introduction, the rippled sheet remained largely a theoretical construct for many decades.
Decoding the Rippled Sheet: Raskatov's Pioneering Study
In a pioneering 2021 study, Raskatov and his team made a significant leap in understanding the rippled beta sheet structure. By blending a small peptide with equal parts of its mirror image, they achieved the conjectured structure. However, these structures did not meet the full expectations of the hypothesized extended, periodic rippled beta-sheet layer topography envisaged by Pauling and Corey.
Overcoming Doubts and Unlocking Potential
In their latest study, Raskatov's team ingeniously substituted other amino acids for one of the triphenylalanines. This led to the creation of slightly altered tripeptides and their mirror-images. These new tripeptides enabled the formation of three distinct aggregating peptide systems that formed extended antiparallel rippled beta sheet layers. The results from subsequent X-ray crystallography confirmed that these crystal structures closely matched Pauling and Corey's predictions, thus revitalizing the potential of this unique protein configuration.
This journey into the world of protein structures, brought to you by the freeastroscience.com team, has revealed the exciting potential of the rippled beta sheet. With its newfound promise, the rippled beta sheet could soon unlock new frontiers in material development and biomedical applications.
Reference:
Amaruka Hazari et al, The rippled β-sheet layer configuration—a novel supramolecular architecture based on predictions by Pauling and Corey, Chemical Science (2022). DOI: 10.1039/d2sc02531k
Ariel J. Kuhn et al, A crystal-structural study of Pauling–Corey rippled sheets, Chemical Science (2021). DOI: 10.1039/D1SC05731F
You Might Also Like :

















0 commenti:
Post a Comment